Print version
Search Pub Med
| 019P Nottingham, UK 7th Focused Meeting on Cell Signalling |
Identification of a novel quinoxaline-based small molecule dual glucagon-like peptide-1 receptor and glucagon receptor allosteric modulator
Introduction: Glucagon-like peptide-1 receptor (GLP-1R) and glucagon receptor (GCGR) are attractive treatment targets for Type 2 diabetes mellitus (T2DM)(1). These two Class B G protein-coupled receptors can be activated by various endogenous ligands, such as glucagon, glucagon-like peptide-1 (GLP-1) and oxyntomodulin (OXM, a C-terminal extended form of glucagon); all of which possess insulinotropic actions(2). Current GLP-1R-based treatments involve peptide drugs which are natural or modified version of GLP-1 and these are clinically efficacious(3). However, these only come as injectables, as such suffer from poor patient compliance and higher production cost. Hence, oral GLP-1R treatment therapies have been prompted, leading to the search of small molecule GLP-1R agonists or positive allosteric modulators (PAMs).
Method: BETP(4) and Compound 2(5), which are GLP-1R agonist-positive allosteric modulators, were used as chemical queries against 3 purchasable chemical libraries. A novel quinoxaline-based compound was identified and characterised as a GLP-1R PAM in cAMP bioassays. 9 analogues based on 80% structural similarity of this lead chemical scaffold were further explored. Allosteric activities on GLP-1R and GCGR endogenous ligands were characterised using homogenous TR-FRET LANCE® cAMP detection kit (PerkinElmer®) in Chinese Hamster Ovary-K1 (CHO) cells stably expressing GLP-1R (CHO-GLP-1R), CHO cells stably expressing GCGR (CHO-GCGR) and rat insulinoma (INS-1) gastric inhibitory polypeptide receptor (GIPR) knock-out cells.
Results: Only analogue 249 with a modification on the quinoxaline-ring scaffold, enhanced OXM-mediated cAMP accumulation in GLP-1R in CHO-GLP-1R cells (pEC50 values increased from 8.10 ± 0.07 to 8.71 ± 0.09, p<0.01, n≥ 3, Fig. 1) and INS-1 GIPR knock-out cells (pEC50 values increased from 7.62 ± 0.08 to 8.11 ± 0.13, p<0.001, n≥3, Fig. 2). It also potentiated OXM-mediated cAMP accumulation in GCGR in CHO-GCGR cells (pEC50 values increased from 9.19 ± 0.11 to 10.24 ± 0.09, p<0.0001, n≥3, Fig. 3).
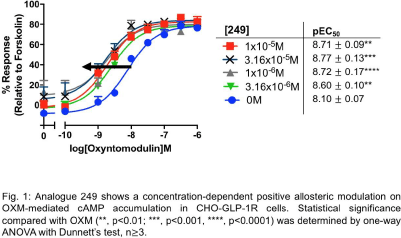
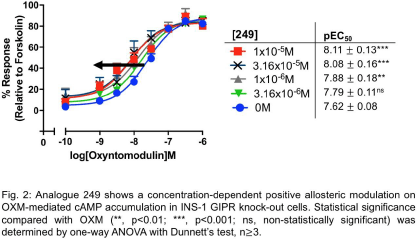
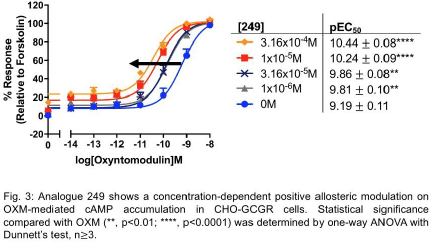
Conclusions: Here we show how a modification on the quinoxaline-ring scaffold affects allosteric modulation in GLP-1R and also in GCGR, an observation that is unseen in other quinoxaline-based GLP-1R PAMs. Hence, analogue 249 may serve as an experimental tool to examine allosteric modulation in these two receptors and a novel OXM-based T2DM treatment.
References:
1. Wewer Albrechtsen, N. (2018). Peptides 100: 42-47.
2. Graaf, C. et al. (2016). Pharmacological Reviews 68: 954-1013
3. Eng, C. et al. (2014). Lancet 384(9961): 2228-2234.
4. Sloop, K. et al. (2010). Diabetes 59: 3099-3108.
5. Knudsen, L. et al. (2007). PNAS 104: 937-942.

