Print version
Search Pub Med
| 027P Nottingham, UK 7th Focused Meeting on Cell Signalling |
The affinity and selectivity of orexin A, orexin B and orexin antagonists for the human orexin 1 and orexin 2 receptors.
Introduction: Orexin A and orexin B are endogenous wake-promoting neuropeptides that activate orexin 1 (Ox1R) and orexin 2 (Ox2R) receptors and a lack of orexin leads to narcolepsy [1]. Orexin agonists are a potential novel treatment for narcolepsy while orexin antagonists may be useful for insomnia. Here we investigate the affinity of several compounds reported to be orexin receptor ligands using 3 different radioligands.
Method: CHO cells stably expressing the human Ox1R or Ox2R were used and whole cell radioligand binding assays performed using 3H-SB674042, 3H-EMPA or 3H-almorexant (2hr, 37°C). Suvorexant (10μM) was used to determine non-specific binding. Radioligand KD values were obtained from saturation experiments and KD values for competing ligands were calculated using the Cheng-Prusoff equation [2].
Results: Saturation binding revealed an affinity for 3H-SB674042 of 4.65±0.28nM (n=15) for the Ox1R, but was too low to determine for the Ox2R. The affinity of 3H-EMPA was 7.86±0.46nM (n=12) for the Ox2R, but was too low to determine for the Ox1R. The affinity of 3H-almorexant was 1.72±0.27nM (n=13) for the Ox1R and 2.68±0.53nM (n=12) for the Ox2R. The experimental window (difference between total and non-specific binding) was however considerably larger for 3H-SB674042 (Ox1R) or 3H-EMPA (Ox2R) than that for 3H-almorexant (Figure 1). Although most ligands fully inhibited specific binding to yield log KD values (Table 1, Figure 2), orexin A and orexin B only partially inhibited the specific binding of 3H-SB674042 and 3H-EMPA at both Ox1R and Ox2R to yield apparent KD values as shown (Figure 2, Table 1).
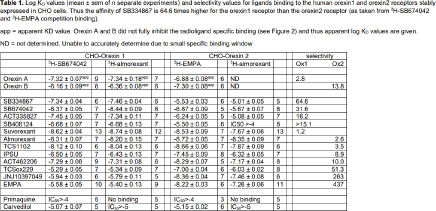
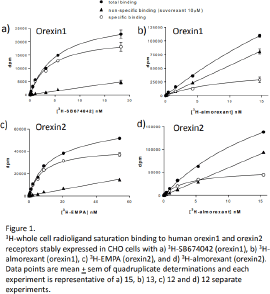
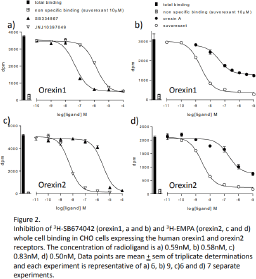
Conclusion: Ligands with Ox1R selectivity (e.g. SB334867 and SB674042) and Ox2R selectivity (e.g. EMPA and JNJ10397049) were identified as well as high affinity non-selective ligands (e.g. almorexant and suvorexant). The larger window of specific binding made 3H-SB674042 and 3H-EMPA better radioligands than 3H-almorexant in this whole cell binding assay. Primaquine and carvedilol, previously reported to have orexin receptor affinity [e.g. 3], had no, or extremely poor affinity only. Whereas most ligands fully inhibited the radioligand specific binding (suggesting competition at the same binding site), orexin A and B were only able to partially displace 3H-SB674042 (Ox1R) and 3H-EMPA (Ox2R) specific binding suggesting that the binding sites of orexin A and B do not fully overlap with those of the radioligands.
References:
[1] Gotter AL et al., 2012 Pharmacol Rev 64: 389-420
[2] Baker JG (2005) Br J Pharmacol. 144: 317-322.
[3] Turku et al., 2016 J Med Chem 59: 8263-8275

