| 030P Nottingham, UK 7th Focused Meeting on Cell Signalling |
Elevation of cAMP through inhibition of phosphodiesterases mediates growth suppression in glioblastoma cells
Introduction: Glioblastoma, a highly aggressive form of brain tumour, is currently incurable. Alterations in cAMP levels appear to be associated with malignancy (1), and thus targeting cAMP levels through selective phosphodiesterase (PDE) inhibition, as well as through other pathways, may be an approach to cancer therapy (2). Here, we investigate whether modulating levels of cAMP may offer a route to growth suppression in glioblastoma.
Methods: In this study rat C6 glioma cells were used as a model for glioblastoma (3), paired with the human embryonic kidney cell line HEK293S as a normal control. Both cell lines were treated with a range of PDE inhibitors, including trequinsin, IBMX, rolipram, amrinone, and zaprinast (10 pM - 100 μM, for 30 minutes), and accumulation of intracellular cAMP was measured using the LANCE cAMP detection kit (PerkinElmer, Boston, MA). Cell proliferation was assessed using the CCK-8 method (Sigma Aldrich, Dorset, UK), with cells being treated for 72 hours with selected PDE inhibitors. Studies using the PKA inhibitor H89 were also performed, to determine whether any observed anti-proliferative effects might be mediated by downstream signalling via cAMP-dependent protein kinase A (PKA).
Results: The C6 glioma and HEK293S cells displayed similar profiles of cAMP accumulation upon treatment with selected PDE inhibitors (Figure 1), with trequinsin and amrinone showing greater Emax compared to other compounds tested. PDE inhibitors not only triggered cAMP accumulation but also showed anti-proliferative effects. The adenylyl cyclase agonist forskolin, as well as the PDE inhibitors, significantly suppressed the growth of the rat glioma cells, although the human HEK293S cells appeared to be less responsive to PDE inhibition, with rolipram actually causing increased proliferation (Figure 2). The PKA inhibitor H89 reversed the anti-proliferative actions of forskolin on the glioma cells.
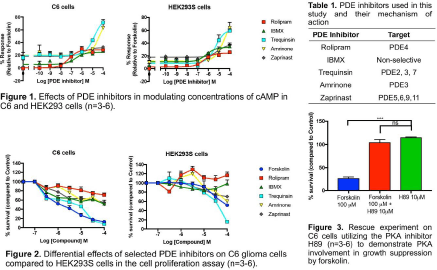
Conclusions: Trequinsin (a non-selective PDE2/3/7 inhibitor) was the most potent compound tested, modulating cAMP and inhibiting cell proliferation in both C6 glioma and HEK293S cells. Accumulation of cAMP appeared to accompany growth suppression in the glioma cells, but since several PDEs known to be expressed in glioblastoma cells also hydrolyse cGMP, the involvement of cGMP in modulating glioblastoma cell growth also needs to be addressed.
References:
(1) Sengupta et al. (2011). Trends Pharmacol Sci, 29: 647-654
(2) Savai et al. (2010). Expert Opin. Investig. Drugs, 19: 117-131
(3) Grobben et al. (2002). Cell Tissue Res, 310: 257-270

