Print version
Search Pub Med
| 031P Nottingham, UK 7th Focused Meeting on Cell Signalling |
Identification of the first small molecule positive allosteric modulator of the Gastric Inhibitory Polypeptide Receptor
Introduction: Peptide-based incretin mimetics are limited by poor oral bioavailability. Orally active small molecules that can modulate incretin receptor pharmacology are far more attractive therapeutics. Indeed, small molecule glucagon-like peptide-1 receptor (GLP-1R) positive allosteric modulators (PAMs) have been developed that modulate GLP-1R activity to promote insulin secretion (1). There are, as yet, no small molecule allosteric modulators of the gastric inhibitory polypeptide receptor (GIPR). In-silico docking identified 11 compounds predicted to bind the GIPR and an initial screen revealed that one compound (C9) had potential activity.
Method: Mammalian HTRF-based second messenger assays for cAMP accumulation and intracellular calcium ((Ca2+)i) mobilisation were utilised to measure the response of HEK-293 and CHO-K1 cells stably expressing the GIPR, and GLP-1R CRISPR KO INS-1 832/3 cells to GIP (1-42) or GIP (Pro3) in the presence and absence of C9. Glucose-stimulated insulin secretion (GSIS) assays were performed to investigate the effect of C9 on GIP (1-42) potentiation of insulin secretion from isolated human pancreatic islets. The operational model of allosterism was used to determine the allosteric cooperativity factor (log(αβ)) for C9 at the GIPR (1). Data are given as mean±SEM (n individual repeats) and analysis was performed using Students t-test or one way ANOVA and Dunnetts post-test where appropriate.
Results: The potency of GIP (1-42) or GIP (Pro3) to stimulate cAMP accumulation was significantly increased when cells were costimulated with C9 (Table 1) and resulted in an allosteric cooperativity factor for C9 greater than 1 (log(αβ); 1.09±0.11 and 1.28±0.28, respectively). This indicated that C9 was a PAM. 15 minutes pre-treatment of GIPR expressing HEK-293 cells with C9 produced a significant increase in Emax (3.05 fold, p<0.01) and pEC50 (p<0.05) for GIP (1-42) mediated ((Ca2+)i) mobilisation. Pharmacological analysis of C9 was then extended to GLP-1R KO INS-1 cells where costimulation with C9 significantly increased the pEC50 of GIP (1-42) to stimulate cAMP accumulation (Table 1 , logαβ; 1.13±0.82). Furthermore, 1 hour pre-incubation of isolated human pancreatic islets with C9 resulted in a dramatic enhancement of GIP (1-42) potentiation of GSIS (Figure 1).

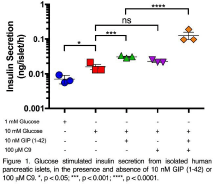
Conclusions: We have identified the first small molecule PAM of the GIPR that can potently enhance cAMP accumulation, ((Ca2+)i) mobilisation and importantly, insulin secretion. The roles that GIPR plays in blood glucose homeostasis and bone formation suggests that C9 may have therapeutic benefits for patients with type 1 or type 2 diabetes, or osteoporosis.
References:
(1). Wootten D et al. (2012). Mol Pharmacol 82(2): 281-290.

