Print version
Search Pub Med
| 0037P Nottingham, UK 7th Focused Meeting on Cell Signalling |
Identification of proteomic changes in primary human bronchial epithelial cells in response to chronic formoterol treatment
Introduction: Bronchial asthma is characterised by bronchoconstriction, airway hyper-responsiveness and elevated mucus secretion. Pathophysiological changes observed in asthma involve altered expression of inflammatory mediators and reflect genetic heterogeneity and environmental influences (1). Current therapies include long-acting β2-adrenoceptor (β2-AR) agonists (LABAs), which exploit the bronchodilatory action of β2-ARs on airway smooth muscle cells (2). Short-term use of these treatments causes beneficial airway relaxation, but chronic use exacerbates asthma symptoms (2-3). Identification of proteomic changes in response to chronic LABA treatment could therefore provide insight into the molecular mechanisms involved in LABA-induced exacerbations and might allow the design of more effective asthma therapies.
Method: Primary human bronchial epithelial cells (HBEC) were subjected to the long-acting β2-AR agonist formoterol (100 nM) for 48h. Cells were lysed and subjected to SWATH-MS (label free quantification by data independent acquisition; 4) to identify differentially expressed proteins. The identified proteins were processed for network analysis using MetaCoreTM pathway analysis suite and the formoterol-mediated changes in expression of proteins present in the network were confirmed by western blotting. Furthermore, to investigate changes in protein expression following more chronic β2-AR stimulation, HBECs were subjected to formoterol (100 nM) for 7 days and protein expression evaluated by western blotting. One-way ANOVA followed by Dunnett's post-hoc test was employed to assess statistical significance.
Results: SWATH-MS analysis identified 9 differentially expressed proteins in response to formoterol treatment in HBECs. Network analysis revealed that the top ranked network included calreticulin, peroxiredoxin-1 and macrophage migration inhibitory factor (MIF). Expression of these three proteins under control and formoterol-stimulated conditions at 48 h and 7 days were confirmed using western blotting (Table 1).
Table 1: Calreticulin, peroxiredoxin-1 and MIF expression in HBECs under control and formoterol (100 nM; 48h and 7 day)-stimulated conditions. *p<0.05 and **p<0.01 versus control cells. Data represent means ± S.E.M. obtained from three independent experiments.
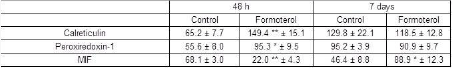
Conclusion: Calreticulin and Peroxiredoxin-1 are both up-regulated in HBECs following 48h exposure to formoterol, but return to control levels within 7 days. In contrast, MIF is down-regulated at 48h, but substantially up-regulated upon more chronic exposure to formoterol. These changes in MIF levels could contribute to asthma exacerbations observed upon chronic use of formoterol.
References:
1. Koczulla AR et al. (2017). Drug Discov Today 2: 388-396.
2. Billington CK et al. (2017). Handbook of Experimental Pharmacology 237: 23-40.
3. Walker J et al. (2011). British Journal of Pharmacology 163: 18-28.
4. Huang Q et al. (2015). Proteomics 15:1215-23.

