Print version
Search Pub Med
| 038P Nottingham, UK 7th Focused Meeting on Cell Signalling |
Real time kinetic analysis of β2 adrenoceptor β-arrestin2 recruitment using NanoBiT luciferase complementation
Background: G protein coupled receptors (GPCRs) activate multiple intracellular effectors, including G proteins and β-arrestins (1). Some ligands bias GPCR signalling between pathways, and often this has been proposed to reflect distinct receptor conformations (1). However, apparent signalling bias may also arise through different ligand-receptor kinetics (2), thus requiring kinetic analysis of ligand-receptor-effector interactions. Here we have used a novel luciferase complementation (NanoBiT) assay to measure recruitment of β-arrestin2 to the β2 adrenoceptor (3) and investigated its ability to explore the kinetics of agonist and antagonist interactions.
Method: HEK293T cells were transiently transfected (lipofectamine) with SNAP-tagged β2 adrenoceptors with C terminals fused to the large luciferase fragment (LgBiT) and β-arrestin2 with a N-terminal (Sm114) low affinity complementary peptide. 48h later, NanoBiT luminescence assays were performed in HBSS/0.1% BSA at 37°C, with 5min furimazine substrate pre-incubation followed by agonist stimulation. Luminescence was measured throughout agonist stimulation (30min, BMG Pherastar2) at 37°C. Where required, 10min antagonist preincubation was used before furimazine substrate addition. Concentration response curves were fitted in GraphPad Prism v7 for apparent pEC50 and Rmax values, as mean±s.e.m (n=3/4).
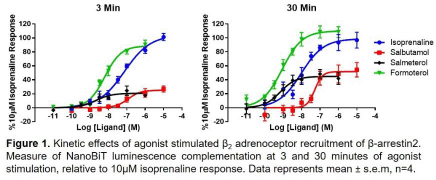
Results: The isoprenaline (10µM) response in the β-arrrestin2 NanoBiT assay peaked at 3 min, with a sustained recruitment component at 30 min, and potency increasing 12-fold between 3 and 30 minutes (pEC50 values; 7.17±0.08 vs 7.85±0.19, respectively; see Figure 1). Formoterol was also a full agonist, relative to isoprenaline, with a 12-fold increased potency after 30 min (pEC50 values; 8.17±0.11 vs 8.90±0.18). Salbutamol and salmeterol were partial agonists, whose relative Rmax values increased between 3 and 30 min (27.0±5.4 vs 60.5±3.3 and 21.9±2.0 vs 53.6±7.5 %10µM isoprenaline; P<0.05, Student’s t-test; see Figure 1). Pre-treatment with ICI118551 (1-30nM) led to non-surmountable antagonism of the isoprenaline response throughout the 30 min agonist incubation. However, while propranolol (0.3-10nM) inhibition of the 3 min isoprenaline response was non-surmountable, surmountable antagonism was observed by 30min, yielding a propranolol pA2 value of 9.57.
Conclusion: NanoBiT complementation reports rapid and reversible β2 adrenoceptor recruitment of β-arrestin2. Its real time measurements demonstrate changes in the relative efficacy of partial agonists over time, and the kinetic effects of slow and fast dissociating β-adrenoceptor antagonists.
References:
(1) Stott L.A., et al. (2016). Biochem Pharmaco, 101:1-12.
(2) Herenbrink C.K., et al. (2016). Nature communications, 7:10842.
(3) Dixon A.S., et al. (2016). ACS Chem Biol, 11:400-408.