| 044P Nottingham, UK 7th Focused Meeting on Cell Signalling |
Actions of ticagrelor on vascular smooth muscle cells
Introduction: The reversible P2Y12 inverse agonist ticagrelor is in widespread clinical use as an anti-thrombotic in patients with acute coronary syndrome, showing enhanced efficacy compared to clopidogrel. We have previously demonstrated that ticagrelor displays inverse agonism at the platelet P2Y12 receptor, with an additional inhibitory action on the equilibrative nucleoside transporter 1 (ENT1) which further enhances platelet cyclic AMP (cAMP) intracellular accumulation[1]. Although ticagrelor has been extensively characterised in the context of antiplatelet therapy, there has been less work examining ticagrelor-dependent actions elsewhere within the cardiovascular system. Here, we sought to characterise P2Y receptor expression in venous and arterial vascular smooth muscle and identify potential actions of ticagrelor in these cells.
Method: Human saphenous vein was obtained with ethical approval from coronary bypass surgeries and smooth muscle cells (HSV-SMC) isolated by explant culture, aortic smooth muscle cells (Hao-SMC) were obtained from Promocell. Samples of freshly isolated saphenous vein smooth muscle were snap-frozen and stored for RNA extraction. P2Y12, P2Y1, P2Y13 and smooth muscle phenotype markers were assessed by Taqman qPCR. Cultured cells were serum-starved for 48 h before all treatments. Migration was assessed using Incucyte scratch wound assay. Data were analysed by t test or one- or two-way ANOVA.
Results: P2Y12 expression was detected in all freshly-isolated and cultured saphenous vein samples (n=13), with expression reduced following culture to P1 when compared to fresh isolates (7.02±2.615 (n=4) vs. 100±37.4 %, (n=6)) with corresponding shift from contractile to synthetic profile as assessed by MYH11 expression. Smooth muscle cell scratch wound migration at 8 h was significantly reduced by treatment with ticagrelor (10μM, DMSO), but not AR-C66096 (10μM), in both cell types (Fig. A, n=4-6). Ticagrelor-mediated inhibition of HSV-SMC migration was unaltered in the presence of the adenosine receptor antagonist xanthene amine congener (XAC; 1μM, DMSO) (Fig.B, n=5). *=p<0.05, **=p<0.01, ****=p<0.0001.
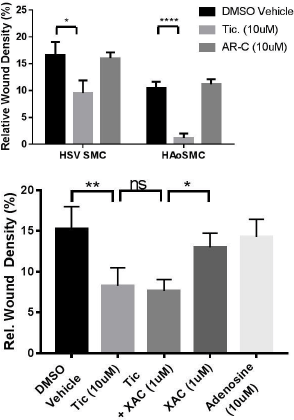
Figures: A. SMC migration at 8 h in the presence of P2Y12 inhibitors ticagrelor (10μM) and AR-C66096 (10μM). B. SMC migration at 8 h in the presence of XAC (1μM) +/- ticagrelor (10μM), versus adenosine (10μM)
Conclusion: Vascular smooth muscle cells possess P2Y12 receptors and display reduced migration in response to ticagrelor but not the P2Y12 antagonist AR-C66096. This effect was maintained in the presence of adenosine receptor antagonism indicating that ticagrelor is able to regulate vascular smooth muscle function independent of ENT1 inhibition, potentially through inverse agonist action at the P2Y12 receptor.
References:
[1] Aungraheeta et al. (2016). Blood 128(23):2717-2728.

