| 051P Nottingham, UK 7th Focused Meeting on Cell Signalling |
Use of fluorescence correlation spectroscopy following SMALP based protein extraction in the study of ABC transporter pharmacology
Introduction: ABCG2 is a human ATP-binding cassette (ABC) transporter that influences pharmacokinetics and bioavailability of multiple drugs. Progress in understanding ABCG2 pharmacology has been mostly limited to studies in non-native insect cell expression systems1. In this project we have used styrene-maleic acid copolymer lipid particles (SMALPs) to isolate ABCG2 in nanodiscs from a mammalian system. SMALP-ABCG2 particles were characterised by fluorescence correlation spectroscopy (FCS) to enable in vitro study of ABCG2 pharmacology.
Methods: Fluorescently tagged ABCG2 was solubilised by SMALP-based extraction from HEK293T cells2 and purified via an N-terminal polyhistidine tag. Samples were analysed by FCS using a ZEISS LSM510 Confocor 3 using 488nm or 633nm excitation, with 30-100s reads in a ~0.2-0.5fL confocal volume, calibrated as described3. Autocorrelation data were fitted to 1 or 2-component 3 dimensional models including a triplet state, whilst molecular brightness was determined by photon counting histogram analysis (PCH) of the same data (bin time = 20 μs). All data are reported as mean±SD for n=3 independent experiments with statistical significance indicated by one-way ANOVA followed by Sidak post-hoc analysis (**=P<0.01).
Results: Molecular brightness of SMALP encapsulated GFP-ABCG2 (SMALP-ABCG2) indicating dimeric complexes by comparison to monomeric (CD86) or dimeric (CD28) membrane protein controls (Figure 1A). A fluorescent native drug substrate, BODIPY-Prazosin, showed a fast diffusion coefficient in the absence of SMALP-ABCG2. In the presence of 20-30nM transporter, an additional BODIPY-prazosin species was observed with a much slower diffusion coefficient equivalent to SMALP-ABCG2, indicating a transporter bound fraction (Figure 1B).
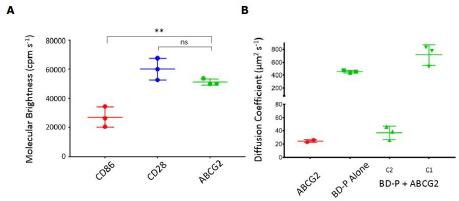
Figure 1. Measurements from PCH analysis (A) indicate the molecular brightness of GFP-ABCG2 is equivalent to CD28 (dimeric control), and significantly higher than that of CD86 (monomeric control). In binding assays using BODIPY-prazosin (BD-P, 500nM; B), presence of SMALP-ABCG2 leads to the appearance of a second, slower diffusing “bound” component (C2) in addition to free ligand (C1). The C2 diffusion coefficient corresponds to that measured for SNAP-Surface® Alexa Fluor® 647 labelled SNAP-ABCG2 in SMALP particles (red).
Conclusion: Dimeric ABCG2 can be extracted from cells in a maintained membrane environment as demonstrated by PCH analysis. FCS provides a mechanism to quantify relative concentrations of free and bound fluorescent drug substrate. This represents a novel platform for investigating transporter binding and recognition mechanisms.
References:
1. Horsey, A.J. et al. (2016). Biochemical Society transactions, 44(3), pp.824-30
2. Gulati, S. et al. (2014). Biochem J, 44(0), pp.1-24
3. Ayling, L.J. et al. (2012). Journal of Cell Science, 125(4), pp.869-886.

