Print version
Search Pub Med
| 008P London, UK Current trends in Drug Discovery – Young Scientists and Tomorrow’s Medicines |
Methods for collection and quality assessment of pharmacokinetic data
Introduction Physiologically Based Pharmacokinetic (PBPK) models are now widely accepted as quantitative biological models useful for simulating the time-dependent disposition of chemicals (or their metabolites) in animals and humans and boost the scientific basis for human health risk assessments. The aims of the current work were to present methods used for collecting and ensuring the quality of chemical-specific parameters required for PBPK model development for case study chemicals in the EU-ToxRisk project.
Methods Data collection proceeded with a variety of publicly available sources identified such as PBPK databases1, reference books2, and literature reviews3. These were accessed through manual extraction and performing website scrapes with a python script. To address data quality assessment, comprehensive guidelines for pharmacokinetics (PK) data collection were developed. These guidelines were then applied to the reported chemical-specific parameters measured in both in vitro and in vivo tests and a comprehensive dataset was created for a defined list of chemicals in the project.
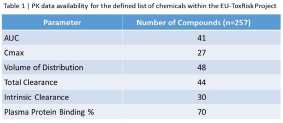
Results The present work resulted in guidelines for quality assessment of publicly available PK data that allow for consistency in data quality, as well as reduction in bias due to personal judgement calls. Furthermore, the work resulted in identification of data gaps for the EU-ToxRisk case study chemicals (the total number of chemicals was 257). In Table 1, the number of metadata for the chemicals included are detailed. These data have been validated and will be used in development and testing of PBPK models for these chemicals.
Conclusion The present work has addressed some of the major problems in the PK parameters data collection process:
I) The created guidelines helped ensure the collection of high quality data to be used in model development.
II) When multiple data sources were available for a unique chemical, the developed guidelines were crucial in the selection of preferential data sources.
III) Data gaps in PK chemical-specific parameters for a specific set of chemicals were identified.
References
(1) ChEMBL Database (EMBL-EBI), https://www.ebi.ac.uk/chembl/
(2) Baselt, R. C. (2017). Disposition of Toxic Drugs and Chemicals in Man (11). Biomedical Publications.
(3) Obach, S. R., Lombardo, F., & Waters, N. J. (2008). Drug Metabolism and Disposition 24, 1385-1405
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 681002.

