Print version
Search Pub Med
| 009P London, UK Current trends in Drug Discovery – Young Scientists and Tomorrow’s Medicines |
Translational understanding of the cardiovascular risk due to alpha2A-adrenoceptor antagonism
Introduction. Early detection of off target molecular pharmacology plays a key role in reducing attrition rate due to safety issues during later stages of the drug discovery and development process. In vitro profiling of compounds against a broad range of targets that may cause an adverse drug reaction (ADR) in humans, allows for the early detection of undesirable molecular pharmacology and ability to predict ADRs1. Testing of Compound A in such a promiscuity panel allowed for early detection of α2A-adrenoceptor antagonism as an off-target liability. α2A-adrenoceptor antagonism carries cardiovascular (CVS) risks of hypertension, resting tachycardia and vasodilation. These haemodynamic effects can be attributed to action through centrally and peripherally expressed α2A-adrenoceptors, the former regulating central noradrenergic output and the latter controlling vascular tone and cardiac sympathetic activity. Compound A is considered to be peripherally restricted therefore we posed the question ‘is the CVS risk different for a peripherally-restricted versus a centrally-penetrant α2A-adrenoceptor antagonist?’ and could we use this information to enable an informed decision on the progression of Compound A.
Methods. A translational analysis to understand the consequences of peripherally-restricted (MK-467 also known as L-659 068) versus centrally penetrant (Yohimbine, Mirtazapine) α2A-adrenoceptor antagonism was conducted to contextualise the safety liability. For reference compounds data was extracted from the literature.
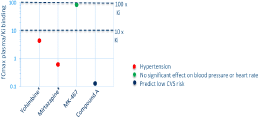
Results. For the centrally penetrant antagonists hypertension occurred at low multiple cover of α2A-adrenoceptor Ki (0.65-4.4x) whereas for the peripherally restricted antagonist no significant effects on blood pressure were seen at a high multiple cover (85x) (Figure 1). This implies hypertension is driven predominantly through a centrally driven mechanism. Applying this information to compound A, which has a target coverage of <1x Ki in plasma, the risk of hypertension was deemed low and negated the need for in vivo evaluation.
Conclusion. Central exposure predominates in driving haemodynamic effects due to α2A-adrenoceptor antagonism. This translational understanding can be applied to other compounds when assessing off-target α2A-adrenoceptor antagonism risk negating the need for animal studies to investigate. Figure 1. Target coverage required clinically for a peripherally restricted (MK-467) versus a centrally penetrant (Yohimbine and Mirtazapine denoted by *) α2A-adrenoceptor antagonist to drive haemodynamic changes.
References
1. Bowes et al., 2012. Nat Rev Drug Discov. 11: 909-22

