| 003P Edinburgh, United Kingdom Pharmacology Futures 2018 – celebrating 250 years of pharmacology in Edinburgh |
Non-invasive in human in vivo target engagement biomarkers to strengthen GO/NO-GO decisions
Introduction: Only about 1 in 10 compounds entering phase I reaches market authorization and eventually the patient. The highest attrition rate is caused in the proof-of-concept phase due to lack of efficacy and/or safety in the target population1. Development of the capsaicin model proved to be useful for evaluating target engagement and dose selection of calcitonin gene-related peptide (CGRP)-(receptor) antagonists in early clinical drug development2. Likewise, we developed 2 new non-invasive, safe and reproducible target-engagement biomarkers for potential future analgesics: the transient receptor potential Ankyrin-1 (TRPA1) channel (activated by cinnamaldehyde) and the pituitary adenylate cyclase-activating polypeptide type I receptor (activated by maxadilan). These are referred to as the cinnamaldehyde (CA) and maxadilan (MAX) model, respectively.
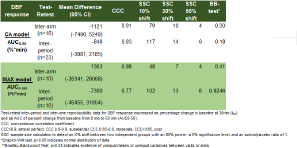
Methods: For both models, dose finding and reproducibility assessments were performed during single-centre, open-label, placebo-controlled studies in healthy subjects. For CA (n=11): 3%, 10%, and 30% cinnamaldehyde (CA) and placebo (=vehicle) were topically applied on the forearm. For MAX (n=10): 0.9, 3, 10ng maxadilan and placebo (=vehicle) were injected intradermally in the forearm. For both models, reproducibility between arms and between periods was assessed. CA and MAX induced dermal blood flow (DBF) was assessed by laser Doppler imaging (LDI) at baseline and every 10 minute post-challenge. To assess reproducibility, the concordance correlation coefficient (CCC) and Bradley- Blackwood test (BB-test) were used. In addition, sample size calculations (SSC) were performed to evaluate the use of the model in future clinical studies comparing active treatment with TRPA1 or PAC1 antagonists with placebo treatment.
Results: For both CA and MAX, all 3 doses increased DBF compared to vehicle at all time-points. 10% CA and 0.9ng MAX were chosen as effective and safe doses to evaluate the reproducibility. DBF responses to 10% CA and 0.9MAX were found to be reproducible between arms (CCC>0.7) and visits (CCC>0.7). Based on SSC, these models allow to detect a change in DBF of 30-50% between 2 independent groups of maximum 10-15 (CA) and <10 (MAX) subjects with 80% power.
Conclusions: In order to tackle the high attrition rate in clinical drug development, “the future of pharmacology” should consider implementing more target-engagement biomarkers, such as the CA and MAX model, early in the development of new drugs, to guide go/no-go decisions.