| 009P Edinburgh, United Kingdom Pharmacology Futures 2018 – celebrating 250 years of pharmacology in Edinburgh |
Surrogate outcomes supporting conditional marketing authorisations and accelerated approvals by the european medicines agency, 2011-2018
Introduction: In situations of unmet medical need, Conditional Marketing Authorisation (CMA) and Accelerated Assessment (AA) pathways are fast-track routes enabling early European Medicines Agency (EMA) marketing approval. CMA is conditional on completing regulator-imposed post-marketing studies. AAs require complete safety and efficacy data whilst shortening regulatory assessment time but have no imposed conditions. Surrogate outcomes (or endpoints), intended to predict desired clinical outcomes, reduce drug development time1. Pivotal studies supporting both CMAs and AAs commonly report surrogate outcomes, especially biomarkers. Non-validated surrogates may not reflect intended clinical outcomes. Subsequently, drugs may not provide intended benefits. We determined the numbers of CMAs and AAs granted according to clinical and surrogate outcomes, and assessed whether surrogates were validated. Fleming and Powers propose an outcomes hierarchy: Level 1 is a clinical outcome; Level 2, a validated surrogate; Level 3, a non-validated surrogate; Level 4, an un-established correlate2. Ciani et al. also propose a hierarchy: Level 1 requires controlled trial evidence that surrogate and clinical effects correspond; Level 2 requires ‘consistent association’ between surrogate and clinical outcomes across observational studies; Level 3 requires biological plausibility1.
Methods: European Public Assessment Reports (EPARS) were used to assess CMAs and AAs issued between January 2011 and January 2017. Ethics approval was not required. PubMed searches were used to identify validated surrogate outcomes. Search terms were: [‘endpoint’] and [validat* surrogate outcome OR validat* surrogate endpoint OR validat* surrogate end-point] and [‘indication’]. Fleming and Ciani hierarchies were used to determine surrogate validity. Fleming ‘Level 2’ and Ciani ‘Level 1’ were judged validated surrogates. Other levels were judged non-validated.
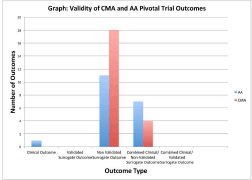
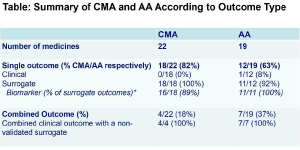
Results: 22 and 19 medicines were authorised through CMA and AA respectively (Table, Figure). 16/22 CMAs and 6/19 AAs were granted for malignant conditions. 6/22 CMAs and 13/19 AAs were granted for non-malignant conditions; 7/13 non-malignant condition AAs were for hepatitis C (HCV) medicines, all based on a non-validated biomarker, ‘sustained virological response’. Most surrogate outcomes were biomarkers (Table). 13/41 (32%) EPARs did not discuss or justify the pivotal trial outcome.
Conclusions: Non-validated surrogate outcomes, most of which are biomarkers, commonly support authorisation decisions. There is inherent uncertainty with non-validated surrogate outcome use. Post authorisation obligations imposed on CMAs address this to some extent. For AAs, medicines approvals granted on the basis of non-validated biomarker outcomes should be re-evaluated as clinical efficacy evidence emerges.
References:
(1)Ciani O et al. (2017). Value in Health 20:487-495.
(2)Fleming TR, Powers JH.(2012).Stat Med 31:2973-2984.