| 012P Edinburgh, United Kingdom Pharmacology Futures 2018 – celebrating 250 years of pharmacology in Edinburgh |
Atorvastatin activates skeletal ryanodine receptors: Design of next-generation statins
Introduction We have recently reported that simvastatin can activate single skeletal muscle ryanodine receptor (RyR1) channels, stimulating sarcoplasmic reticulum (SR) Ca2+ release from isolated skeletal muscle cells.1 We suggested that the interaction of statins with RyR1 may contribute to their muscle-related side effects, including fatal rhabdomyolysis. The aim of this study was to investigate whether this effect was also seen with atorvastatin, a more recently developed and, in contrast to simvastatin, fully synthetic drug.
Methods Sheep skeletal heavy SR membrane vesicles containing RyR1 channels were incorporated into planar phospholipid bilayers under voltage-clamp conditions as previously described.2 Atorvastatin sodium salt was added to the cytosolic or luminal channel side at the concentrations indicated. Binding of [3H]ryanodine to RyR1 was also used as an indirect measure of the open probability (Po) of populations of channels in their native membranes. Heavy SR vesicles were incubated for 90 min at 37°C with 200 μg of protein/ml. Nonspecific [3H]ryanodine binding was determined in the presence of 1000-fold excess unlabelled ryanodine.
Results Low concentrations of cytosolic atorvastatin, at 10 μM free Ca2+, significantly and reversibly increased Po from 0.029±0.0098 (mean±SEM, n=22) in control conditions to 0.077±0.023 (mean±SEM, n=16, p<0.05) with 100 nM atorvastatin and 0.083±0.027 (SEM, n=13, P<0.05) with 1 μM atorvastatin. The addition of atorvastatin to the luminal (trans) side of RyR1 did not activate the channels even at high concentrations (≤100 μM). Atorvastatin also significantly increased [3H]ryanodine binding to sheep skeletal SR membranes.
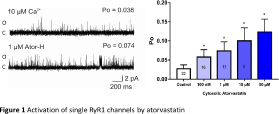
Conclusions Cytosolic application of atorvastatin reversibly activates single skeletal RyR1 channels in a concentration-dependent manner and does so at concentrations low enough to be clinically relevant. Addition of luminal atorvastatin had no effect, indicating that atorvastatin interacts with RyR1 via cytosolic binding sites. We therefore suggest that RyR1 channel activation is a common property of clinically relevant statins, and that removal of this interaction will lead to the development of a statin drug with reduced muscular side-effects. We now aim to utilise a medicinal chemistry approach to design a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor that can effectively lower blood cholesterol levels yet does not activate RyR1.
References
1) Venturi, E. et al. (2018) Br J Pharmacol 175(5): 938-9522
2) Sitsapesan, R. et al. (1991) J Physiol 434: 469-488

