| OC002 Virtual Meeting BPS & SMR joint meeting: Current Trends in Drug Discovery |
Impact of Standard of Care Parkinson's Disease medications on Off-time readouts in clinical trials. A Quantitative Systems Pharmacology (QSP) approach
Introduction/Background & aims There is increasing interest to use wearable-based digital health measures to continuously monitor the effect of therapeutic interventions on motor symptoms in Parkinson’s Disease (PD). Apps like Apple’s MPower2 offer the possibility to generate a more relevant clinical outcome, for instance on Off-time (i.e. the duration of time in a day when symptoms are not well controlled by medication) for patients day-to-day in clinical trials with novel therapeutic interventions. However, this outcome is partially dependent upon the pharmacokinetic profile of the L-dopa formulation and adjunctive therapies administered. The aim of this study was to mitigate clinical variability of Off-time with various therapeutic combinations.
Method/Summary of work We used a combination of pharmacokinetic and pharmacodynamic modelling to generate daily profiles of clinical Off-time for realistic formulations of various approved Standard of Care medications (Sinemet, Stalevo, Rytary and Duodopa). The pharmacodynamic modelling is based on an advanced QSP computer model of human basal ganglia representing different subregions of the motor circuit with the beta/gamma ratio of local field potential in the subthalamic nucleus as a proxy silico readout for rigidity and bradykinesia[1]. This model was previously calibrated using the Unified Parkinson's Disease Rating Scale (UPDRS) Part III scale. The beta/gamma threshold for Off-time was determined using published clinical trial data on the impact of various L-DOPA formulations on Off-time reported by PD patients[2]. The probability of Off-time below the threshold was also included.
Results/Discussion We report the observed difference between immediate and extended release of the carbidopa-levodopa formulation and reductions in Off-time of other PD medications.
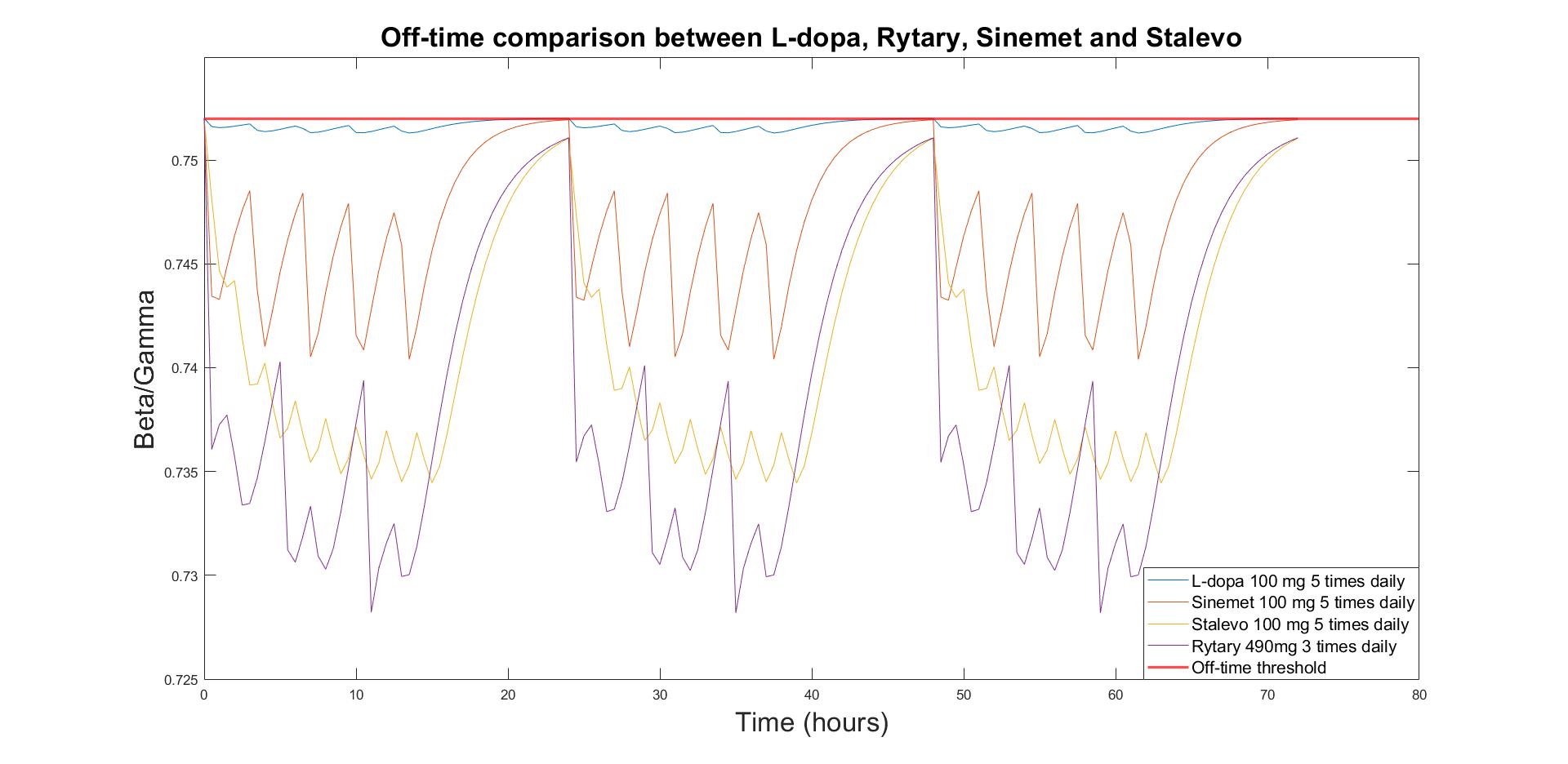
Figure 1 - Beta/Gamma ratio for different formulations of L-dopa
Our simulations demonstrate that the accumulated duration of Off-time during the day heavily depends upon the L-dopa formulation and nature of the adjunctive therapy (reduction by ~5% in Beta/Gamma ratio with L-dopa/A2A antagonist combination compared with L-dopa alone).
Conclusion(s) This approach allows for testing the impact of various comedications in combination with novel therapeutic interventions in clinical trials and clinical practice. This QSP approach can identify and mitigate sources of variability in clinical readouts of Off-time, relevant to trials using wearable-based digital health measures.
Reference(s)
[1] Roberts P, Spiros A, Geerts H. A Humanized Clinically Calibrated Quantitative Systems Pharmacology Model for Hypokinetic Motor Symptoms in Parkinson’s Disease. Front Pharmacol. 2016;7.
[2] Hauser R, Hsu A, Kell S et al. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson's disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol. 2013;12(4):346-356.

