Articles
- The slopes of concentration-effect (dose-response) curves or Clark, Gaddum, Schild, and pA2
- Physiology and Pharmacology at the University of Manchester
- Paul Lechat (1920-2003)
Issues
Volumes
VOLUME 1 - ISSUE 2 - THE SLOPES OF CONCENTRATION-EFFECT (DOSE-RESPONSE) CURVES OR CLARK, GADDUM, SCHILD, AND PA2
The development of pharmacology between the two world wars owes much to the need to measure the activity of drugs, which had not been obtained chemically pure, in terms of international standards. Originally this included substances such as digitalis, thyroxine and insulin and even in 1947 extracts of curare alkaloids ('Intocostrin') were standardised by bioassay. An important reference book was J.H.Burn's 'Biological Standardisation' (Oxford Medical Publications, 1937) and students in his laboratory rapidly learnt that the slopes of dose-response curves were all different - and that you needed a preparation with a steep dose-response curve* to make a sensitive bioassay.
Differences between Pharmacology and Biochemistry: Agonists
The fact that dose-response curves varied so much made pharmacology different from biochemistry. According to Michaelis-Menten kinetics the substrate, S, and enzyme, E, combined one-to-one forming the complex, ES, and the velocity of the reaction, V, was directly proportional to ES, rising from zero to a maximum, V max. The concentration of substrate producing a half-maximal rate, Km (the Michaelis constant), half-saturates the enzyme and is the dissociation constant for the process E + S<->ES.
The relation can be written
V = VmaxS/(S + Km)(1)
and when V is plotted against log[S] the curve is sigmoid with central part approximately linear and with a fixed slope: to go from V = 25% to V = 75% of Vmax, S must be increased 9-fold. Pharmacologists working with tissues found that a similar change from 25% to 75% of the maximum response was often produced by much smaller increases: sometimes doubling the concentration was sufficient. The activity of a substrate for an enzyme was determined by Km and Vmax. In contrast, the concentration of a drug which produced a half-maximum response from a tissue, EC50, was not constant: with powerful agonists a maximum response was produced from the activation of only a small proportion of receptors.
* This term was used in the older work: it has since been replaced by 'concentration-effect' curve.
Antagonists:
(i) effects expressed as a fraction of control responses
For biochemists studying the effects of an inhibitor, I, competing with a substrate, S, for an enzyme, the concentration of enzyme-substrate complex, ES, is:
ES = (S/Km) /[1 + (S/Km) + (I/Ki)] (2)
where Ki is the dissociation constant for the process E + I <->EI. The reaction rate is directly proportional to ES, so if this is measured for different concentrations of S and I and Km is known, it is possible to estimate the inhibitor constant, Ki. The same relation applies to the drugs A (agonist) and B (antagonist) acting on tissue receptors (Gaddum, 1937), but with tissues the response obtained is not directly proportional to receptor occupancy (the receptor equivalent of ES). So although pharmacologists can obtain concentration- effect curves for an antagonist by measuring its effect as the inhibition of control responses from the agonist on the tissue, the position and shape of these depend upon the concentration of agonist used and the shape of its concentration - effect curve. A 50% reduction in the response to a concentration of agonist where the curve is steep represents a much smaller antagonism than for an agonist where it is flat.
(ii) effects expressed in terms of concentrations producing the same response
The idea of measuring the effects of an antagonist in terms of how much you needed to increase the agonist to restore the response to its uninhibited size appeared in a paper by Clark and Raventos (1937: Table 1).
Antagonism of acetylcholine (Ach) and Me4N+
Preparation
|
|
Rat intestine |
Frog auricle |
Frog rectus |
|||
|
antagonist |
Ach |
Me4N+ |
Ach |
Me4N+ |
Ach |
Me4N+ |
|
Atropine |
-8.1 |
-7.9 |
-8.3 |
-7.7 |
-4.2 |
-3.8 |
|
OctN+Me3 |
-5.5 |
-5.0 |
-5.0 |
-4.6 |
-4.1 |
-4.0 |
|
Bu4N+ |
-3.6 |
-3.7 |
- |
- |
-3.2 |
-3.0 |
|
'curarine' |
inactive |
inactive |
-5.1 |
-4.5 |
-6.8 |
-6.5 |
|
Figures indicate the logarithm of the molecular concentration of antagonist which necessitated a 10-fold increase in the concentration of Ach or Me4N+ in order to obtain the same effect: 'curarine' was a crude extract of alkaloids. For competition the numbers should depend on the tissue and the antagonist and not on the agonist. |
||||||
Clark died in 1941 but after the war Schild (1947), using home-made automated equipment, showed how antagonist activity could be measured in this way. The responses to the agonist in the presence and in the absence of antagonist are the same (it is a 'null' method) so ES or agonist-receptor occupancy in equation 2 will be the same and the relation between this and the response will not matter. He coined the phrase 'dose-ratio'* to express the effect of a particular concentration of an antagonist. For a dose-ratio X, the response produced by [A] of agonist (dissociation constant KA) is also produced by X[A] in the presence of [B] of antagonist (dissociation constant KB), so
(A/KA)/[1 + (A/KA)] = X(A/KA)/[1 + X(A/KA) + (B/KB)]
or X = 1 + (B/KB)(3)
This is often called the Gaddum-Schild equation and shows that the concentration of antagonist which necessitates doubling the concentration of agonist (X = 2) to obtain the same size of response should be KB in the process B + R <-> BR.The symbol pA2 is the logarithm of the reciprocal of this concentration, log.[1/KB], a positive number which should be independent of the agonist, providing it acts at the receptor R. It is exactly the same as biochemical pharmacologists measure in binding experiments with receptor material and an inhibitor competing with a radio-labelled ligand. In these, too, the dissociation constant for the inhibitor should be the same for all ligands and that particular receptor.
* I have kept this term although 'concentration-ratio' is in present use.
Relation between dose-ratio and antagonist concentration (Schild plots)
In practice it is often easier to measure higher effects than dose-ratios of 2: it is also more accurate because errors affect [dose-ratio - 1]. Clark and Raventos worked with dose-ratios of 10. If a range of concentrations of antagonist is tested, the graph of log.[DR-1] against log.[B] - a 'Schild' plot - should be a straight line with a slope of unity if the agonist and antagonist act competitively (Arunlakshana and Schild, 1949). As in biochemistry, antagonism in pharmacology is not restricted to competition. Some compounds bind covalently to a receptor. When used against an agonist with very high efficacy - i.e. which produce a maximum response even when only a small proportion of receptors is activated - these 'irreversible' antagonists give linear Schild plots over a range. In pharmacology it may also be possible to obtain the opposite effect from a tissue by a separate mechanism, for example to antagonise contraction by causing relaxation. This has been called 'physiological' antagonism*. A check that agonist and antagonist are acting competitively can be made by testing them along with another known competitive antagonist.
* The term 'inverse agonist' is used to describe the situation where the two drugs act on the same receptor and the receptor mechanism can operate in either direction.
Uses
The measurement of the activity of an antagonist as a log.equilibrium constant, whether it is called pA2 or log.[1/KB] has obvious advantages in the testing of antagonists. It is directly proportional to the free energy of binding (-![]() G = RT lnKB) and errors in its measurement appear to be normally distributed. As well as for comparing different antagonists, it can be used for comparing the types of receptors in different tissues or for checking that the binding characteristics of receptor material obtained from membranes are the same as for receptors in functional tissues.
G = RT lnKB) and errors in its measurement appear to be normally distributed. As well as for comparing different antagonists, it can be used for comparing the types of receptors in different tissues or for checking that the binding characteristics of receptor material obtained from membranes are the same as for receptors in functional tissues.
Measuring the steepness of concentration-effect curves
The relation between effect, E, and concentration, A, can be represented:
E = M [A]P/([A]P + [A50]P)(4)
where M is the maximum, [A50] produces half this, and the exponent, P, is a convenient measure of the steepness. Parker & Waud (1971) used this as an empirical model for concentration - effect curves but it has the same form as the equation used by Hill (1913) to describe the binding of oxygen to haemoglobin: P is the Hill coefficient. The steepness can be estimated from the ratio of the concentrations producing 75%, [A75], and 25%, [A25], of the maximum: [A75]/ [A25] = 91/P but Parker & Waud showed that with iterative calculations using a computer it was possible to fit values of E by least-squares directly to values of A and obtain estimates of M, P and [A50]. For one-to-one binding P=1 and the relation becomes the same as equation (1). The exponent might indicate the molecularity of the process. For P=2 the process might be: 2A+R<->AR: for P=0.5 it might be: A+2R<->RAR, but, as with the binding of oxygen to haemoglobin, the situation is unlikely to be as simple as this. Nevertheless values of P>1 indicate that the signal generated by an agonist combining with a receptor is amplified, and values of P<1 that it is reduced.
What you find experimentally
Values of P were calculated from figures appearing in the British Journal of Pharmacology (vols 120-123: Barlow, 1999). The selection was arbitrary but the distribution appeared to be log.normal: the coefficient of skewness was 5.0 for values of P (kurtosis 52) compared with -0.04 (kurtosis 4.5) for values of log.P, and skewness = 0 (kurtosis 3) for a normal curve. The results are summarized in Table 2.
TABLE 2
Values of the exponent, P
|
|
range |
sd |
mean |
log. mean |
median |
n(*) |
|
1 |
Binding experiments |
|||||
|
|
0.18-1.30 |
0.26 |
0.74 |
0.68 |
0.75 |
51 (8) |
|
2 |
Measurements of electrical events, e.g.currents |
|||||
|
|
0.29-2.33 |
0.54 |
1.21 |
1.09 |
1.04 |
41 (4) |
|
3 |
Measurements of second messengers |
|||||
|
|
0.25-3.67 |
0.65 |
1.00 |
0.86 |
0.84 |
64 (9) |
|
4 |
Positive effects on tissues, e.g. contraction |
|||||
|
|
0.33-8.02 |
0.88 |
1.12 |
0.99 |
0.97 |
81 (3) |
|
5 |
Negative effects on tissues, e.g. relaxation or inhibition |
|||||
|
|
0.20-2.12 |
0.43 |
1.05 |
0.96 |
0.96 |
118 (6) |
|
* number with P<0.5 |
||||||
For binding experiments (1) P should be 1and there are many examples in the literature (outside the survey) where this is so. In this sample there was no value greater than 1.3 but a long 'tail' of values less than 1, with 8 out of 51 (16%) having P<0.5. Some of the flat curves involved two binding sites and with others it appeared that the baseline was greater than zero.
In groups 2 and 3 the events measured should be closer to the binding step than those in groups 4 and 5. From the range it appears that amplification of the signal can occur and the biggest effects are with positive effects on tissues (4). The sample, however, has not included some curves which were known to be very steep long before computer methods were available for estimating P. The inhibition of contractions of the phrenic nerve-rat diaphragm preparation by (+)tubocurarine chloride is an example. Re-examination of some old results gave P=3.74, greater than the biggest value for negative responses (group 5 in Table 1) but it is known that the compound competitively antagonizes neurally released acetylcholine. Why is the curve so steep? The shapes and position of curves produced by competitive antagonists depend upon the size of the agonist effect used in the experiments - this can be called the 'degree of agonist stimulation' and expressed as A/A50. When the antagonist opposes a small effect the value of P is close to 1. When a large effect is used the curve is shifted towards higher concentrations and P is that for the agonist. In the experiments with (+)tubocurarine chloride it appears that neural stimulation produces a very high agonist effect indeed with A/A50 approximately = 12 (Barlow, 1995). If antagonist concentration - inhibition curves are obtained where each concentration of antagonist was tested against both a low (A/A50< 1) and a high (A/A50>1) degree of agonist stimulation it is possible to calculate antagonist dissociation constants, K B (Barlow et al. 1997). This could be a useful alternative to measuring dose-ratios when the antagonist has limited solubility.
Operational windows
(i) agonists
The steepness of concentration-effect curves may depend upon the way the effect is measured. In order to study this parallel experiments were made with contractions recorded isotonically (proportional to the change in length) and isometrically (proportional to the change in tension), using tissues from the same animal and with the same load or initial tension (Barlow et al, 2001a). For contraction of the rat uterus in oestrus by carbachol* the mean values of P (n 10) were 7.7 (se 1.9, isotonic) and 1.9 (se 0.2, isometric). In experiments with guinea-pig ileum mean values (n 13) were 2.49 (se 0.22, isotonic) and 1.73 (se 0.19, isometric) for carbachol: for histamine (n 7) they were 1.74 (se 0.20, isotonic) and 1.29 (se 0.14, isometric). In all these experiments the curves with isotonic recording were also displaced towards lower concentrations. Examples are shown in Figure 1. An explanation for the higher values of P with isotonic recording is that the 'operational window' - between what is recorded as zero and as maximum - is narrower. It is possible to record increases in tension with the tissue held at a fixed length when the muscle cannot shorten further because of its bulk.
(ii) antagonists
Many of the curves in group 5 are for substances, e.g. relaxants or dilators, physiological antagonists which activate separate receptors producing the opposite effects to constrictors. These are particularly likely to be affected by the operational window of the recording system. With isometric recording it will be possible to measure decreases in tension beyond the point where the tissue is fully extended under the load. When relaxation curves were obtained for (-)isoprenaline opposing the effects of carbachol or histamine on guinea-pig ileum, values of IC50 were lower with isotonic than with isometric recording in parallel experiments (Barlow et al, 2001b). The curves were steeper and there were wider shifts in antagonist concentration before the point was reached where increasing the concentration of agonist produced a contraction which could not be overcome by the antagonist. To standardise methods for comparing drugs and avoid the complications arising from the shape of the agonist curve, the effects of antagonists can be compared against agonist concentrations which on their own produce a half-maximum effect.
(iii) windows and signal amplification
The idea of operational windows can be extended from recording systems to cellular events close to the response. The highest value in Table 1, P=8 (Shiraishi et al 1998), is for the relation between tension in the middle cerebral artery of the rabbit and the internal concentration of calcium ions. There is a narrow window between concentrations which produce no effect on tension and those which produce the maximum: narrow windows produce steep concentration - effect curves. If the signal curve generated by the previous step is represented by equation 4 and only the points lying within the window are fitted to this expression, it is possible to see that the curve is not symmetrical. If there is a threshold, the lower half will be steeper than the overall fit and the upper half flatter. The high values of P indicate selection rather than amplification of the signal. Lew and Flanders (1999) call this 'threshold inertia' and it should be particularly important for the effects of physiological antagonists which probably ultimately act by raising or lowering the internal concentration of calcium ions.
* Widely used for the assay of adrenaline (which causes relaxation) before spectrophotofluorimetric methods were invented.
Future Developments
This article was inspired by the choice of 'pA2' as a title replacing 'BPS Bulletin' and the need for Graeme Henderson to explain pA2 for the benefit of 'members of the society who have entered pharmacology from another discipline'. It is such people, anatomists, biochemists, biophysicists, chemists, pathologists, pharmacists, physiologists, statisticians and, above all, medically qualified people who in the past have made pharmacology what it is. Gaddum referred to a pharmacologist as a Jack (Jill)-of-all-trades and it is important to retain this outward-looking approach. Perhaps what distinguishes pharmacologists is that they do not limit the compounds they test to those which occur naturally and that they are concerned with comparing the activities of different compounds, so far as is possible. The wide variation in the slopes of concentration - effect curves is a particular feature of pharmacology which makes this difficult and the title pA2 is a fitting tribute to past work on the problem of measuring the activity of competitive antagonists. It is to be hoped, however, that this will be seen in context: much more remains for the future.
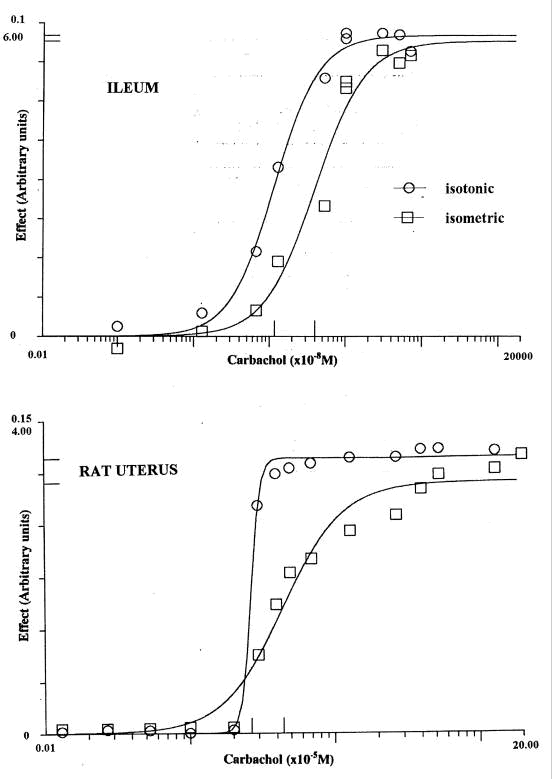
Figure 1. Concentration-effect curves for carbachol with samples of adjacent tissue from guinea-pig ileum and rat uterus in oestrus recorded isotonically and isometrically. The scales have been chosen so that the graphs are of roughly comparable height with the maxima indicated on the ordinate: values of EC50 are marked on the abscissa.
ReferencesArunlakshana, O. & Schild, H.O, (1949) Some quantitative uses of drug antagonists. Br. J. Pharmacol., 14, 48-58.
Barlow, R.B. (1995) The use of an antagonist for estimating the degree of agonist stimulation during physiological release. Trends Pharmacol. Sci., 16, 262-264.
Barlow, R.B. (1999) A survey of the slopes of concentration- response curves. Br. J. Pharmacol., 128, 301P.
Barlow, R.B., Susan M. Bond, Elizabeth Bream, Lisa Macfarlane, & McQueen, D.S. (1997) Antagonist inhibition curves and the measurement of dissociation constants. Br. J. Pharmacol., 120, 13-18.
Barlow, R.B., Susan M. Bond, Claire Grant, D.S.McQueen & Zeenat Yaqoob (2001 a) A comparison of effects measured with isotonic and isometric recording: I Concentration- effect curves for agonists. Br. J. Pharmacol., 133, 1081-1086.
Barlow, R.B., Susan M. Bond, Claire Grant, D.S.McQueen & Zeenat Yaqoob (2001 b) A comparison of effects measured with isotonic and isometric recording: II Concentration - effect curves for physiological antagonists. Br. J. Pharmacol., 133, 1087-1096.
Clark, A.J. and Raventos, J. (1937) The antagonism of acetylcholine and of quaternary ammonium salts Quart. J. Exp. Physiol., 26, 375-392.
Gaddum, J.H. (1937) The quantitative effects of antagonistic drugs. J. Physiol., 89, 7P-9P.
Hill, A.V. (1913) The combinations of Haemoglobin with Oxygen and Carbon Monoxide I. Biochem. J., 7, 471-480.
Lew, M.J. & Flanders, S. (1999) Mechanisms of melatonin-induced vasoconstriction in the rat tail artery; a paradigm of weak vasoconstriction. Br. J. Pharmacol., 126, 1408-1418.
Parker, R.B. & Waud. D.R. (1971) Pharmacological estimation of drug-receptor dissociation constants. Statistical evaluation. I Agonists J. Pharmacol. Exp. Ther., 177, 1-12.
Schild, H.O. (1947) pA, a new scale for the measurement of drug antagonism. Br. J. Pharmacol., 2, 189-206.
Shiraishi, Y, Kanmura, Y. & Itoh, T (1998) Effect of cilostazol, a phosphodiesterase type III inhibitor, on histamine-induced increase in [Ca2+] i and force in middle cerebral artery of the rabbit. Br J.Pharmacol., 123, 869-878.
R. B. Barlow
