Articles from this issue
- The plague of plagiarism
- Pharmacology at University College London
- Neuronal pathways involved in reward and addiction
- A Mentoring Scheme for Female BPS Members
Volumes
Issues
VOLUME 3 - ISSUE 3 - NEURONAL PATHWAYS INVOLVED IN REWARD AND ADDICTION
For thousands of years, a wide range of psychoactive drugs has been used for their stimulant, euphoric and rewarding properties. Indeed, the Inuit people are the only human society with no documented recreational drug use, a situation that changed with European contact. In modern times, it has become clear that many of these rewarding drugs can result in compulsive drug intake and addiction. The health and social costs of drug addiction are considerable. For instance, the 2004
The mesolimbic dopamine system, the central
‘reward’ pathway
The first key insight into identifying a neuronal pathway involved in reward came from the pivotal work of Olds and Milner (1954). By implanting electrodes to stimulate discrete parts of the rat brain, they found that rats would lever press over 6000 times per hour to stimulate the medial forebrain bundle. The medial forebrain bundle consists of a population of myelinated fibres that project to the ventral tegmental area (VTA). A significant proportion of VTA neurones are dopaminergic and project to the nucleus accumbens, known as the mesolimbic pathway. Further work showed that for stimulation of the medial forebrain bundle to be rewarding, the mesolimbic pathway had to be intact, and dopamine release in the accumbens had to occur. This led to the theory that the dopaminergic, VTA-accumbens pathway, or mesolimbic pathway, plays a central role in reward.
The role of the mesolimbic pathway in the rewarding effects of drugs of abuse was then unravelled. Firstly, it was found that several drugs of abuse (eg. amphetamine, cocaine, opiates) increased self-stimulation of the medial forebrain bundle. In 1988, Di Chiara & Imperato elegantly showed that administration of a range of abused drugs (nicotine, ethanol, amphetamine, cocaine, opiates) all resulted in an increase in dopamine release in the nucleus accumbens. Furthermore, ‘natural’ rewards such as food seeking and intake, and sexual behaviour have been shown to involve dopamine release in the nucleus accumbens, leading to the idea that drugs of abuse hijack the implicit reward mechanism needed for normal animal behaviour. Although different drugs of abuse all appear to increase dopamine release in the nucleus accumbens, their dissimilar pharmacology means that they act in very different ways, and at different loci within the mesolimbic pathway. This is illustrated in Figure 1.
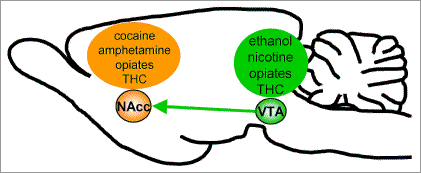
Figure 1. The mesolimbic pathway. Sagittal rat brain section showing the dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAcc) that play a central role in the rewarding effects of drugs. Of the major drugs of abuse, cocaine and amphetamine act at the nucleus accumbens. Nicotine and ethanol increase the firing rate of VTA neurones. Opiates and THC act primarily at the VTA but also have some effects at the NAcc.
Cocaine and amphetamine both inhibit re-uptake of dopamine; additionally, amphetamine also causes release of dopamine from nerve terminals. These two drugs therefore act at the nucleus accumbens. Opiates and tetrahydrocannabinol (THC), the active principle in cannabis, act on m-opioid and CB1 receptors, respectively. In the mesolimbic pathway, these receptors are located on GABAergic interneurons in the VTA, so that activation of these receptors causes disinhibition of the dopaminergic projection neurones. To a lesser extent, activation of m-opioid and CB1 receptors on GABAergic neurones in the nucleus accumbens can cause disinhibition at the dopaminergic nerve terminal. Nicotine acts on nicotinic receptors located somatodendritically on dopaminergic VTA neurones to directly increase their firing rate. The pharmacology of ethanol is more complex, but the predominant effect of ethanol in the mesolimbic pathway is to directly increase the firing rate of dopaminergic VTA neurones through a number of mechanisms including actions at ion channels, GABAA and NMDA receptors. Despite diverse pharmacology, all of these drugs of abuse cause dopamine release in the nucleus accumbens.
Drug addiction: synaptic
plasticity, and beyond the mesolimbic pathway
Although the mesolimbic pathway is heavily implicated in both ‘natural’ (eg. food, water, sex), and ‘un-natural’ (eg. drugs of abuse) reward, activation of this pathway is not sufficient for drug-seeking behaviour. Dopamine release is not thought to drive drug-seeking behaviour, merely to gate such behaviour (reviewed in Wise, 2004, and Salamone et al, 2005). Indeed, the role of dopamine release in the accumbens is more subtle and complex than merely being released as a result of intake of rewarding drugs.
Fascinating human brain imaging studies, pioneered by Volkow and colleagues (reviewed in Lingford-Hughes, 2005) have shown that dopamine is released in the nucleus accumbens of a recovering addict merely as a result of the subject being presented with a drug-related cue, not by the drug itself. The mesolimbic pathway can be seen as a link between the limbic and motor systems, and is interconnected with a number of other brain regions that also play critical roles in drug-seeking behaviour (summarised in Figure 2, and reviewed in Kelley, 2004).
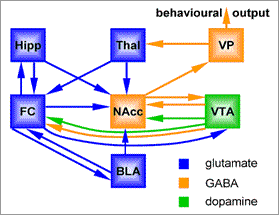
Figure 2. Neuronal pathways involved in drug addiction. Glutamatergic projection neurones are shown in blue, GABAergic projection neurones are orange, dopaminergic neurones shown in green. Abbreviations: Hipp, hippocampus; Thal, thalamus; VP, ventral pallidum; FC, frontal cortex; NAcc, nucleus accumbens; VTA, ventral tegmental area; BLA, basolateral amygdala.
Many drug users do not become dependent or addicted to drugs. However, those that do become addicted display compulsive drug intake, and recovering addicts show a dramatic incidence of relapse, even after prolonged periods of abstinence. Extensive recent work has been performed in order to uncover the cellular mechanisms that might underlie this ‘switch’ between intermittent drug use, and addiction.
Synaptic plasticity (long-term potentiation and long-term depression) is widely thought to be the key synaptic modification underlying learning and memory. Consequently, there have been significant recent advances in the study of synaptic plasticity and addiction, focussing on the mesolimbic pathway, particularly at glutamatergic synapses in the VTA and nucleus accumbens (see Kauer, 2004). Saal et al (2003), echoing the work of Di Chiara & Imperato, found that administration of a single dose of a wide range of addictive drugs (cocaine, amphetamine, nicotine, ethanol, morphine) all caused an enhancement of glutamatergic synaptic strength (long-term potentiation) at VTA neurones. The challenge now is to define the molecular pathways involved in synaptic plasticity caused by drugs of abuse, and how these processes affect network activity within brain regions involved in drug addiction.
Chris Bailey
