Print version
Search Pub Med
Bimatoprost is a mixed inhibitor of the aldo-keto reductase 1C3 (AKR1C3) enzyme: A potential treatment target for sex hormone-dependent diseases
The aldo-keto reductase 1C3 (AKR1C3) enzyme belongs to the aldo-keto reductase superfamily. It has (β/α)8 structure motif and uses β-Nicotinamide adenine dinucleotide (2’-phosphate), reduced NADH/NADPH as cofactors. The enzyme is involved in the reduction of prostaglandin D2 (PGD2), prostaglandin H2 (PGH2), oestrone (E1) and progesterone to 9α,11β-prostaglandin F2 (9α,11β-PGF2), prostaglandin F2α (PGF2α), 17β-oestradiol(E2) and 20α-hydroxyprogesterone, respectively1. Sex hormone dependent diseases, i.e. endometriosis, endometrial cancer, breast cancer and prostate cancer have upregulated AKR1C3 expression, indicating a role in the pathogenesis of these diseases1. Bimatoprost, or 17-phenyl trinor prostaglandin F2α ethyl amide, is a prostaglandin F receptor analogue. It was also found to inhibit the PGD2 11-ketoreductase and PGH2 9,11-endoperoxide reductase activities of AKR1C32. The aim of the project was to investigate whether bimatoprost can also inhibit the catalysis of E1 to E2. The standard assay mixture contained 0.1M potassium phosphate buffer (KPB, pH=6.5), 0.5mM NADP+, 5mM glucose-6-phospate and 1 unit of glucose-6-phosphate dehydrogenase2.1μl ≡ 1µCi of [3H]-oestrone with specific activity of 94Ci/mmol and 1μl of unlabelled oestrone at different concentrations (2.5, 5, 10, 20, 40 and 80μM) were added, in the presence or absence of bimatoprost. The reaction started by the addition of 3μg of recombinant AKR1C3 in a total volume of 50μl for 60 minutes at 37°C which was found to be linear at this time point. The reaction was terminated by 250μl of cold ethyl acetate. 100μl were blotted on a thin layer chromatography plate and developed for 30 minutes in ethyl acetate:chloroform (1:4). The bands were visualised by spraying the plate with methanol:sulphuric acid (1:1) and heated at 110°C for 10min3. The bands for E1 and E2 were scraped off and radioactivity was measured with Packard Tricarb 2100TR scintillation counter.
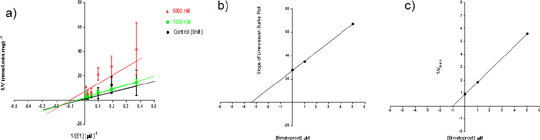
(a) The lineweaver-burk plot of mean reciprocal AKR1C3 velocity (1/V) values (±SD) of 3 experimental repeats showed that bimatoprost is a mixed inhibitor when plotted against reciprocal of oestrone conc. (1/[E1]); (b) a replot of slope vs bimatoprost concentration [µM] was used to estimate Ki value (3.4μM); (c) a replot of 1/Vmax vs bimatoprost concentration [µM] was used to estimate αKi value (1μM). α value is 0.3, indicating that bimatoprost favours uncompetitive inhibition over competitive inhibition.
1 Byrns et al. (2011). J Steroid Biochem Mol Biol 125: 95-104; 2 Koda et al. (2004). Arch Biochem Biophys 424: 128-136 3 Penning et al. (2000). Biochem. J. 351: 67-77
|