Print version
Search Pub Med
| 007P Nottingham, UK 7th Focused Meeting on Cell Signalling |
Development of an ex vivo receptor occupancy assay for the Class C GPCR mGlu5
Introduction: Receptor occupancy (RO) assays play an important role in drug development, as they confirm target engagement in the tissue of interest. We have recently developed HTL0014242, a negative allosteric modulator of mGlu5, and demonstrated RO in rat hippocampus using autoradiography1. However, such methodology requires specialised equipment and thus we have established an ex vivo RO assay using a tissue homogenate filtration-based format. Here, we report assay development and validation using [3H]M-MPEP and HTL0014242 in murine brain tissue.
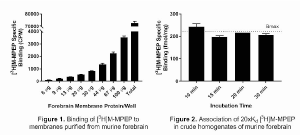
Method: HTL0014242 was synthesized as established in1. Forebrain was dissected from six adult male CD-1 mice. Membranes were prepared from three mice as described previously2 and protein linearity was performed to assess specific binding of [3H]M-MPEP. Using forebrain membranes (30 μg/well), saturation and kinetic [3H]M-MPEP binding experiments were performed. Competition binding assays were used to determine the pKiof HTL0014242. All were carried out as described in1. In RO experiments tissue needs to be rapidly processed to avoid dosed compound dissociation, therefore tissue was prepared by crude homogenisation (Polytron Homogeniser; 7,000 rpm; 20 s) immediately prior to use in protein linearity assays. Using chosen conditions (1.4 mg homogenate/well), the preparation was employed to perform both steady-state saturation and kinetic association assays at 4°C to select conditions resulting in full mGlu5 RO.
Results: Increasing protein concentration correlated with increased specific binding of [3H]M-MPEP and 30 μg forebrain membranes/well resulted in high specific binding without radioligand depletion (Figure 1). Saturation and kinetic binding assays allowed Kd and association/dissociation rates to be calculated, and competition binding assays established that HTL0014242 displays high affinity for the murine mGlu5 (Table 1). By using high [3H]M-MPEP concentrations (20xKd) but short incubation times, it was demonstrated that all mGlu5 receptors in tissue homogenates were occupied by 10 minutes, as calculated by comparing specific [3H]M-MPEP binding to the Bmax determined in steady-state saturation binding studies (Figure 2).
Table 1. mGlu5 ligand binding in murine forebrain. Kd of [3H]M-MPEP was determined by saturation binding (SB) or kinetic binding (KB) assays. Data shown are mean ± SD and as aim was to establish methodology, an n of 2 was performed to limit animal use.
| [3H]M-MPEP | HTL0014242 | |||
|---|---|---|---|---|
| Kd by SB (nM) | Kon (M-1 min-1) | Koff (min-1) | Kd by KB (nM) | pKi |
| 3.91 ± 0.16 | 25,777,542 ± 2,477,593 | 0.0471 ± 0.0262 | 1.79 ± 0.84 | 8.88 ± 0.24 |
Conclusion: Expression of mGlu5 was confirmed in murine forebrain and binding of [3H]M-MPEP and HTL0014242 was characterised. In tissue from dosed animals, performing a 10-minute association of 20xKd [3H]M-MPEP at 4°C would allow the radioligand to occupy all unliganded mGlu5 but should prevent dissociation of HTL0014242. This data will serve as the groundwork for the final HTL0014242 dosing study to confirm mGlu5 occupancy in the murine brain.
References:
1. Christopher JA et al. (2015). J Med Chem. 58: 6653-64.
2. Bradley SJ et al. (2011) Mol Pharmacol. 79: 874-85.

